Controlling Mercury in Cement Manufacture
By Francisco M. Benavides
1. Introduction
The manufacture of cement may emit mercury into the atmosphere if mercury is present in the raw materials or the fuel. To reduce emissions, the United States Environmental Protection Agency (EPA) established the National Emissions Standards for Hazardous Air Pollution or (NESHAP) with the first mercury emission limit set in 2006 at 41µg/dscm at 7% oxygen. However, since 2015, the annual mercury emissions for existing cement facilities are set to 55 lb/million t of clinker (8 – 13 μg/Nm3) and 21 lb per million tons of clinker (3 – 5 μg/Nm3) for new kilns; resulting approximately in 92% reduction in mercury.
To reduce mercury emissions, an in-depth look into all of the stages of the cement process is needed. Controlling the source of the mercury, whether in the raw materials or fuels is the first consideration that needs to be taken. However, if the source can’t be controlled, treatment of the off-gases becomes necessary. Activated Carbon Injection (ACI) is a potential solution but expensive; depending on the amount of mercury present, other solutions can be considered.
2. Mercury Properties
Mercury (Hg) is a natural-occurring element found in limestone and some fossil fuels in the earth’s crust. Mercury may present itself in the form of elemental or metallic mercury, inorganic mercury, and methylmercury as well as other organic compounds. Mercury’s most troubling issue is that elemental mercury is liquid at room temperature, with a low melting point (-38.9°C), and it can evaporate into odorless toxic vapor with a low boiling point of (356.7°C). This means it easily becomes airborne, migrates into water sources and foods especially as methylmercury. Mercury poses very harmful effects to the main functions of the brain, kidney and immune systems.
3. Global Mercury Emissions
Even though the cement industry has decreased mercury emissions over the past few decades, it still remains an environmental concern. According to the 2018 Global Mercury Assessment, cement production accounted for 233,168 kg of mercury emitted, resulting in 11% of global mercury emissions.
Figure 1. Mercury Emissions by Sector
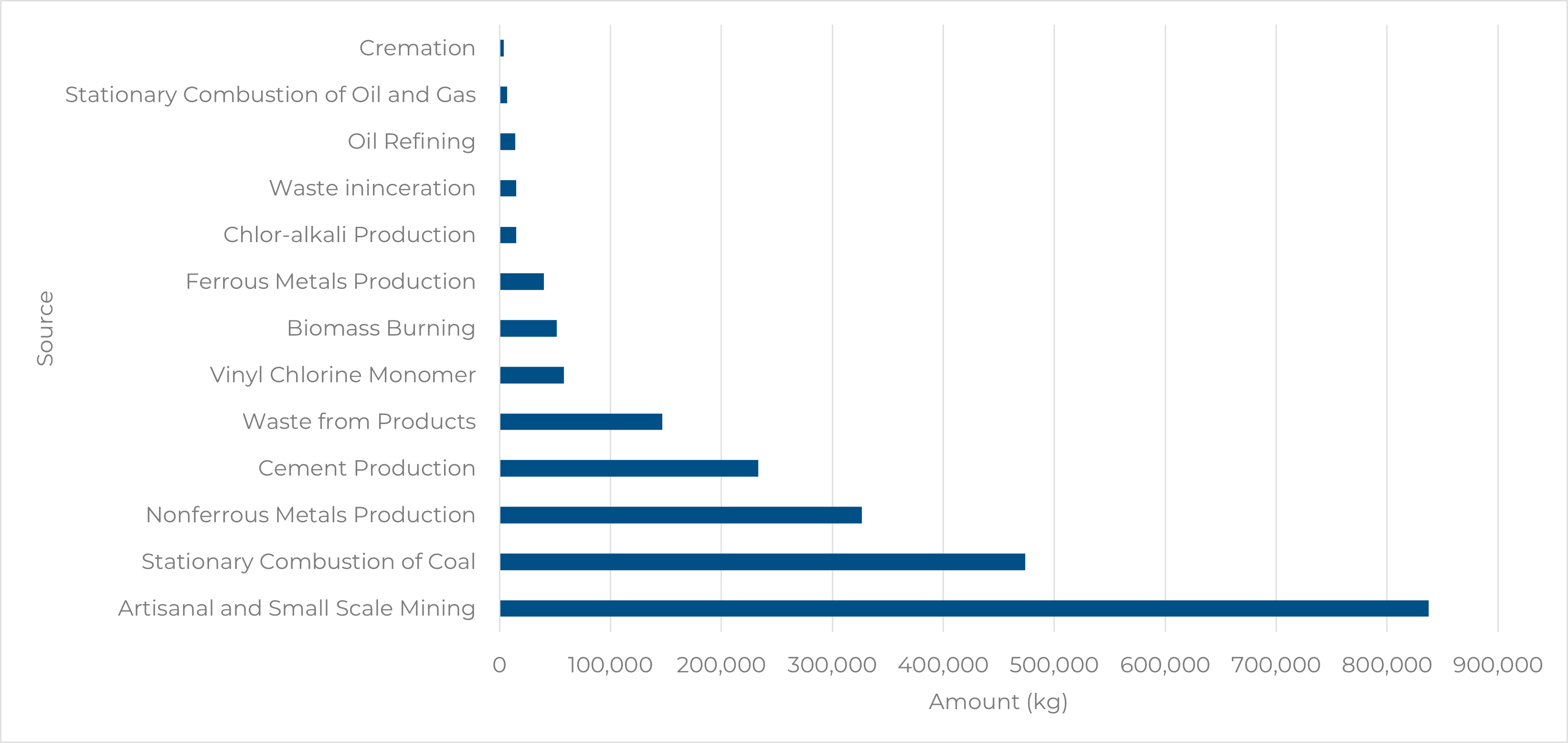
Note. Adapted from Mercury Emissions: The Global Context. (2021, February 1). US EPA. https://www.epa.gov/international-cooperation/mercury-emissions-global-context
4. Technologies to Reduce Hg Emissions in Flue Gases
A Continuous Emission Monitoring System (CEMS) at the stack is used to monitor the emissions from the process.
EPA sanctioned control technologies focus on the emission of gases to the atmosphere, but reducing the mercury content before the pyro-process is a better option, if possible. Testing raw material sources for heavy metals including mercury content should be standard practice when investigating fuel and mineral resources for the manufacture of clinker. Limestone mines in some areas contain mosesite in conjunction with the limestone and this mercury mineral must be removed from the limestone prior to processing.
Mercury particulate captured in the process off-gas baghouse can be encapsulated by a method known in the industry as dust shuttling. The adsorbed mercury in the filter dust is ‘shuttled’ or fed to the cement mill, and eventually encapsulated in concrete.
5. Sorbents
Selection of sorbents and their subsequent capacities derive from many different factors such as: flue gas composition, temperature, residence time of sorbent, mercury concentration in the gas stream, surface area covered by sorbent and species of mercury (i.e., elemental, oxidized). Operation impacts can be possible corrosion from bromine or sulfur impregnated activated carbon and how to handle and dispose of the filtered dust. Controlling the injection system can be complicated and requires constant monitoring and maintenance to meet the environmental emission target.
Powdered Activation Carbon (PAC) is injected into the gas stream after the main baghouse filter; thus, it requires two dust collectors in series. The injection system must be placed upstream enough to meet the required residence time the temperature must be controlled to meet the maximum possible absorption. Lower temperatures are favorable for mercury absorption using PAC, thus seasonal changes in ambient temperature can alter the operations of the PAC system. Controlling the temperature of the exit gas stream is paramount in utilizing the PAC to its full potential, as temperature variations can lead to higher amounts of PAC to meet the regulations required.
PAC is typically halogenated, usually brominated-activated carbon as it enhances absorption of elemental mercury. This brominated-activated carbon can capture up to 90%, but there are limited industrial references. Bromine, however, is corrosive and can cause damage to downstream processes such as bag filters and metals.
When using PAC, control, capture, and disposal of sorbent-laden dust must be declared and approved by the State regulating agency. The dust could be partially shuttled to the cement mill under strict quality controls. The balance needs to be disposed of as a hazardous waste or reprocessed by the supplier of the activated carbon.
The capacity of absorbing oxidized mercury and elemental mercury will differ, and the total concentration of mercury will affect the selection of activated carbon. In the cement industry, the levels of hydrocarbons and other volatiles such as SO2 will need to be considered, as this can lead to reduction of mercury absorption and could prevent PAC from being effective for mercury removal.
Another important factor in mercury capture capacity is surface area and pore size for the activated carbon. However, some studies have shown that saturation such as dispersion and contact time between the reagent and mercury may be more significant.
Table 1. The Specific Surface Areas of Some Absorbent Types

Note. Adapted from Cement Sustainability Initiative. (2016, June). Guidance for reducing and controlling emissions of mercury compounds in the cement industry. World Business Council for Sustainable Development. https://www.wbcsd.org/Sector-Projects/Cement-Sustainability-Initiative/Resources/Guidance-for-reducing-and-controlling-emissions-of-mercury-compounds-in-the-cement-industry
Implementing a PAC solution is capital intensive and other mercury solutions should be considered in combination with the emissions control of other pollutants.
The quantity of activated carbon used can be reduced by blending it with hydrated lime. Operating costs are dependent on the sorbent usage, which can range from 1 to 1.25 dollars per ton of clinker.
6. Kiln Dust Shuttling
Dust shuttling has been identified by the US EPA as a means of reducing the emission of Hg from the clinker plant stack. Thanks to the efficiency of a vertical roller mill (VRM) acting as a scrubber, the raw meal captures the Hg from the gases exiting the kiln preheater. However, as the raw meal is fed to the preheater, the cycle of meal fed to the preheater and the mercury in the meal evaporated by the hot preheater gases keeps concentrating the level of mercury in the gases exiting the preheater. When the raw mill stops operating for maintenance, the mercury in the gases solidifies as the gases are cooled and then collected in the baghouse. This cement kiln dust (CKD) then has a high concentration of Hg that needs to be disposed of, so this dust is “shuttled” to a bin in the cement grinding area to be trickle-metered into the cement mill. The cement containing the Hg will then eventually be incapsulated in concrete.
Since the CKD shuttled to the mill is acting as an additive, partially replacing limestone additive, this method of Hg emission control tends to have an economic benefit in the operating cost as well as solving an environmental issue.
To effectively capture the mercury in the baghouse during VRM-off operations, the gases need to be cooled below 140°C ahead of the baghouse. This is usually accomplished by water evaporation in the gas conditioning tower.
7. Conclusions
The potential for mercury entering the cement plant process is either with the raw materials or fuels. The most likely source could be in the limestone, as it is known that limestones in certain areas contain mercury and, it being the largest component of the raw mix, particular care needs to be taken in sampling and testing the limestone for mercury when analyzing a potential deposit. Raw material additives also need to be tested.
A common source of mercury is in the coal burned in the kiln system, so obtaining an analysis of the heavy metals present in the coal is essential and these pollutants need to be limited in the supply contract.
With the increasing use of alternative fuels, mercury content also needs to be an essential consideration in sourcing these fuels. In an existing operation, with no alternatives, there is no choice but to use abatement technologies to limit emissions, but the best option where possible is to avoid mercury entering the process.
References
- Basic Information about Mercury. (2021, September 24). US EPA. https://www.epa.gov/mercury/basic-information-about-mercury
- Cement Sustainability Initiative. (2016, June). Guidance for reducing and controlling emissions of mercury compounds in the cement industry. World Business Council for Sustainable Development. https://www.wbcsd.org/Sector-Projects/Cement-Sustainability-Initiative/Resources/Guidance-for-reducing-and-controlling-emissions-of-mercury-compounds-in-the-cement-industry
- Gonzaga, E. & Carbonxt Group ltd, USA. (2018, May). Mercury Capture Optimization. International Cement Review, May 2018, 88–90.
- Kogut, K., Górecki, J., & Burmistrz, P. (2021). Opportunities for reducing mercury emissions in the cement industry. Journal of Cleaner Production, 293, 126053. https://doi.org/10.1016/j.jclepro.2021.126053
- Mercury Emissions: The Global Context. (2021, February 1). US EPA. https://www.epa.gov/international-cooperation/mercury-emissions-global-context
- Stewardson, L. (2019, March 6). Optimising Dry Sorbent Injection. World Cement. https://www.worldcement.com/special-reports/06032019/optimising-dry-sorbent-injection/
- World Cement. (2014, January 10). Opportunities for Mercury Abatement – Part 2. World Cement. https://www.worldcement.com/the-americas/10012014/opportunities_for_mercury_abatement_part_2_550/
- Zheng, Y., Jensen, A. D., Windelin, C., & Jensen, F. (2012). Review of technologies for mercury removal from flue gas from cement production processes. Progress in Energy and Combustion Science, 38(5), 599–629. https://doi.org/10.1016/j.pecs.2012.05.001
- International Cement Review (2019, May). PAC for Mercury Control, Dr. Prasanna Seshadri.
- World Business Council for Sustainable Development (WBCSD)/Cement Sustainability Initiative (CSI), July 2016.
About the Author(s)
Francisco M. Benavides, P.E.
Mr. Benavides, Principal Consultant at PEC Consulting Group LLC, has many years of experience in project management, design and construction. He has conducted bankable feasibility studies, economic analysis of mineral transport alternatives, plant valuations for acquisitions and for financing purposes, and due diligence studies. He holds an MBA from Kellogg School of Management, Northwestern University, Evanston, Illinois; a Bachelor of Science and Professional Degree in Civil Engineering from the Missouri University of Science & Technology, Rolla, Missouri; and has completed Graduate Studies in Engineering Management and Environmental Engineering at Missouri University of Science & Technology.
PEC Consulting Group LLC | PENTA Engineering Corporation | St. Louis, Missouri, USA
How can we help you? Get in touch with our team of experts.
